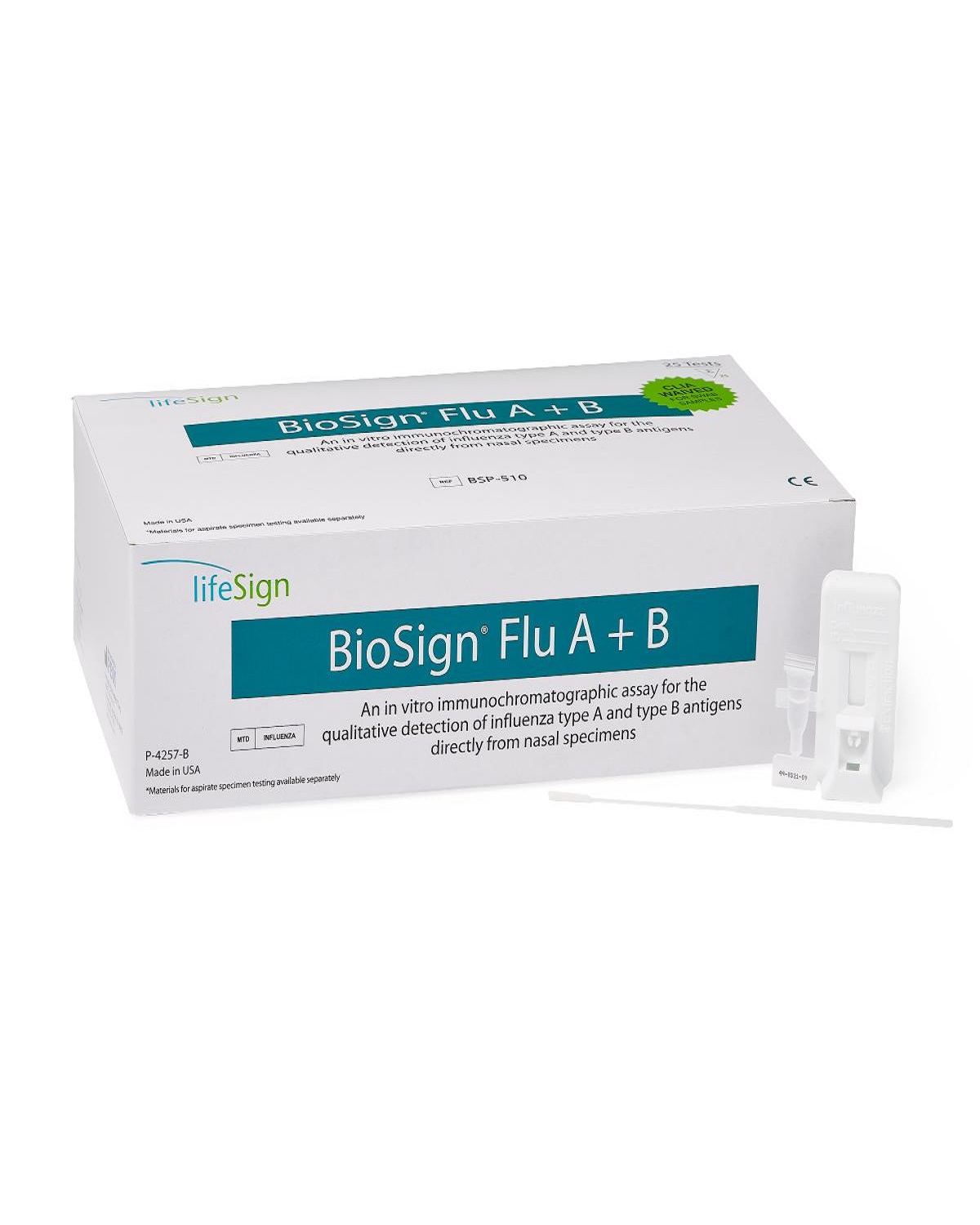
Description
BioSign Flu A & B Test is an in vitro immunochromatographic assay for the qualitative detection of influenza type A and type B antigens directly from nasal specimens.
CLIA Waived.
Kit Contents:
25 test devices
25 extraction reagent capsules
25 sterile swabs
1 positive and 1 negative control swabs
Description
Differentiates between Influenza Type A and B, on a single test device
Produces results in 10 to 15 minutes
Each test device includes built-in controls
Each kit comes with external controls
Meets FDA performance criteria for influenza tests stetted in code 21 CFR 866.3328
CLIA Complexity: Moderate complexity when used with nasal wash/aspirate samples
CLIA Waived when used with nasal and nasopharyngeal swabs
ITEM #BSP-510


Reviews
There are no reviews yet.